Danger of personal import of ED remedies!
 The threat of counterfeit medicines is increasing.
The threat of counterfeit medicines is increasing.
This is a serious threat to your safety.
Individual import of dangerous ED treatment (from the results of a joint survey of the four pharmaceutical companies)
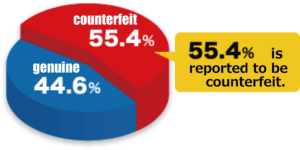
55.4% of Internet distribution (Japan: 43.6%, Thailand: 67.8%) were counterfeit products. The quality of counterfeit products varies, and the content of pharmaceutical ingredients is not only higher or lower than the approved dose, but also contains another ingredients, other ingredients, or multiple impurities.
* Pfizer’s information graph is not limited to Viagra, but is the percentage of counterfeit and genuine products containing other ED treatments Levitra and Cialis.
Private import
Regulations on imports of pharmaceutical products, etc.
Since pharmaceuticals, quasi-drugs, cosmetics and medical devices have a direct impact on human health and body, their quality, effectiveness and safety have been confirmed based on scientific data, etc. It is strictly regulated by the Pharmaceutical Affairs Law so that only new products are distributed in Japan. General individuals can import (so-called individual imports) only when they use it for themselves, and they are not allowed to sell or give away personally imported products to other people. Hmm. The reason why ordinary individuals can import medicines is because of consideration when it is necessary to continue the drug treatment received in a foreign country or when travelers from overseas carry it as a regular medicine.
Intellectual property infringement and personal import
Counterfeit ED drugs brought in from overseas infringe intellectual property rights. Bringing counterfeit products into Japan is prohibited. If you import it individually or take it home from abroad, you may be punished. In addition, ED drugs obtained from overseas through irregular routes (other than medical institutions) are highly likely to be counterfeit products, and unexpected health hazards are expected.
When imported for use by doctors, etc. for treatment
Personal import of medicines, etc. by medical professionals is “when there is a therapeutic urgency and alternatives are not distributed in Japan, and the imported medical professionals are responsible for their own patients. Only for the purpose of “providing for diagnosis or treatment”. We consider such imports by doctors to be “individual medical professionals” imports (type 1), but we do not do so because the following drug side effect relief system does not apply.
Health hazard cases (Singapore / February-May 2008)
Serious adverse events such as coma due to hypoglycemia occurred in patients who took counterfeit ED treatments and three other Chinese herbal medicines (so-called energetic agents). As of May 2008, there were 40 confirmed patients and 87 suspected patients. Four of them died (two of them died from counterfeit ED treatments). * Pfizer information
Counterfeit Viagra manufacturing site
An example of a counterfeit Viagra manufacturing site (China)

Fake Viagra Factory
Counterfeit Viagra is manufactured all over the world. To date, counterfeit Viagra has been found in 60 countries around the world, including Japan. The counterfeit Viagra manufacturing site is unsanitary, as in this case, and there are problems with quality control in the distribution channels. (Sent in bulk in a suitable plastic bag, etc.) (Counterfeit Viagra tablets are bottled in a non-quality controlled location (such as in an apartment)) Sent from a US address It cannot be said that the incoming Viagra is genuine. (Forged Viagra has been confirmed to be imported into Japan via the United States.) * Pfizer information
Counterfeit Viagra Photo

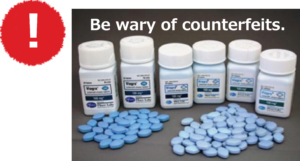
The fake is very elaborate, and it is difficult to distinguish it unless you are an expert. Not only Viagra, but also Levitra and Cialis are rampant. * Pfizer information
Relief system for phytotoxicity
If a genuine ED treatment drug approved by the Ministry of Health, Labor and Welfare is used properly under the prescription of a doctor, but serious side effects occur, the drug side effects will be relieved. The system is a system in which public institutions provide relief from medical expenses. It is funded by pharmaceutical company contributions and state subsidies. In other words, the responsibility is generally not the doctor who prescribes it, but the pharmaceutical company and the Ministry of Health, Labor and Welfare who have given permission to manufacture and sell. On the other hand, in the case of non-genuine ED treatment drugs, in most cases, the individual doctor has been issued a drug supervision certificate at the local welfare bureau under his jurisdiction for the purpose of prescribing to patients from overseas. In other words, it was personally imported by an individual doctor from overseas. The Ministry of Health, Labor and Welfare is also calling attention to drugs imported from overseas by individuals.
Eligible for the phytotoxicity relief system (Ministry of Health, Labor and Welfare)
Diseases due to side effects that occurred after May 1, 1980 (for products such as regenerative medicine after November 25, 2014) despite proper use of medicines (those requiring hospitalization treatment) ), Disability (a condition that significantly limits daily life) and death are health hazards covered by the benefits. “Pharmaceuticals” are medicines that have been approved and licensed for manufacturing and marketing, and include all medicines prescribed at hospitals and clinics, medicines requiring guidance purchased at pharmacies and drug stores, and over-the-counter medicines . However, exempted drugs, etc. are separately defined. “Proper use” is basically to be used according to the indications, dosages and precautions stated in the drug container or package insert, but for individual cases, the current medical science・ Judgment is made from a comprehensive point of view in light of the scientific level of pharmacy.
Precautions regarding personal imports – Ministry of Health, Labor and Welfare
Personal importation of drugs, etc.
Ministry of Health, Labor and Welfare medicine food station monitoring guidance and Narcotics Division
for guidance and enforcement of personal import agency business
the Ministry of Health, Labor and Welfare pharmaceutical Director pharmaceutical onset No. 0828014
Q and A relating to personal importation of drugs, etc.
Ministry of Health, Labor and Welfare Pharmaceutical and Food Safety Bureau monitoring and guidance – narcotics Division
for Internet sales of OTC drugs
Ministry of Health, Labor and welfare pharmaceutical and food Safety Bureau, Department
warning against the health damage caused by counterfeit drugs
Cialis, Levitra
About unapproved and unlicensed drugs (Ministry of Health, Labor and Welfare)
- Results of the 2015 “Unapproved and Unlicensed Drug Purchase Survey” (announced on September 28, 2017)
- About the result of 2015 “Purchase survey of products sold on the Internet” (announced on September 28, 2017)
- About the result of 2014 “Purchase survey of products sold on the Internet” (announced on March 31, 2017)
- Results of the 2014 “Unapproved and Unlicensed Drug Purchase Survey” (announced on December 22, 2016)
- About the result of 2013 “purchase survey of products sold on the Internet” (announced on December 28, 2015)
- About the result of 2013 “Purchase survey of unapproved and unlicensed drugs” (announced on December 28, 2015)
- Results of the 2012 “Purchase Survey of Internet Sales Products” (announced on June 10, 2014)
- Results of the 2012 “Unapproved and Unlicensed Drug Purchase Survey” (announced on October 22, 2013)
- About the result of 2011 “Purchase survey of products sold on the Internet” (announced on November 13, 2012)
- Results of the 2011 Unapproved and Unlicensed Drug Purchase Survey (announced on June 22, 2012)
- Providing information on French-made silicone bag products for breast augmentation (December 28, 2011)
- Calling attention to health hazards caused by counterfeit Cialis tablets (2nd report) (May 26, 2011)
- Calling attention to health hazards caused by counterfeit Cialis tablets (April 26, 2011)
- About cases of health damage (suspicion) caused by unapproved and unlicensed drugs called “hospital diet” imported from Thailand (October 9, 2009)
- About the discovery of so-called health foods (unapproved and unlicensed drugs) containing pharmaceutical ingredients (thioyldenafil) (announced on December 5, 2008)
- About the discovery of so-called health foods (unapproved and unlicensed drugs) containing pharmaceutical ingredients (thiokina piperifill) (announced on June 26, 2008)
- About the discovery of so-called health foods (unapproved and unlicensed drugs) containing pharmaceutical ingredients (cyclopentinafil and N-octyl nortadalafil) (announced on June 11, 2008)
- About the discovery of so-called health foods (unapproved and unlicensed drugs) containing pharmaceutical ingredients (Norhondenafil) (announced on February 26, 2008)
Results of the 2010 Unapproved and Unlicensed Drug Purchase Survey (announced on May 30, 2011) - Results of the 2010 Unapproved and Unlicensed Drug Purchase Survey (announced on May 30, 2011)
- Results of the 2009 Unapproved and Unlicensed Drug Purchase Survey (announced on July 16, 2010)
- Results of the 2008 Unapproved and Unlicensed Drug Purchase Survey (announced on August 20, 2009)
- Results of the 2007 Unapproved and Unlicensed Drug Purchase Survey (announced on August 26, 2008)
About the result of “Purchase survey of products sold on the Internet” About so-called health foods in which pharmaceutical ingredients (sildenafil and similar ingredients) were detected.
List of ED drugs that need attention – US FDA
Levitra analog
Shinjuku West Clinic Shibuya West Clinic Telemedicine
ED・AGA related pages
Difference between free medical care and insurance medical care
Information of approved medications
Information of unapproved medications
What is “drug price standard not listed”?
Viagra
Propecia
Zagallo
